On October 29, 2019, Hila Bernstein, from the Cambridge Public Health Department, facilitated a conversation about Institutional Review Boards (IRBs), how to determine whether your work needs IRB review, and best practices for evaluators when working with IRB staff to navigate the process. Nearly 20 GBEN members participated in the discussion.
During the roundtable, Hila provided a description of IRBs, how an IRB reviews evaluation studies, consideration for evaluators in working with IRBs, recommendations, then facilitated a Q&A and discussion. Below are a few key summary points from the presentation.
What is an IRB?
An IRB is a committee or group of individuals who review certain risks associated with research studies and, if warranted, provide approval for the study to go forward. IRBs review all components of a research study including protocols, methods, materials, and tools used in the study. A key IRB responsibility is ensure a study meets the following requirements:
- Risks to subjects are minimized.
- Risks to subjects are reasonable in relation to anticipated benefits.
- Selection of subjects is equitable.
- Informed consent will be sought from each prospective subject or legally authorized representative.
- Informed consent will be appropriately documented.
- When appropriate, the research plan makes adequate provision for monitoring the data collected to ensure safety of subjects.
- When appropriate, there are adequate provisions to protect the privacy of subjects and to maintain the confidentiality of data.
Identifying an IRB to Review Your Work
Depending on the funding for your study and the type of organization or company you work for, the requirements and options for identifying an IRB to review your work can be different. Research that is federally funded must be reviewed by an IRB. Internal evaluators may already have an established IRB in their organization; if not, that organization could use another institution’s IRB by obtaining a federal wide assurance (FWA) number. In certain situations, an evaluator may need IRB review at their own institution (for example, large institutions and research centers association with hospitals and universities). Independent evaluators or those without an institutional IRB may need to contract with an external IRB entity.
Is it Research?
An important question to consider before even reaching out to an IRB is considering if your work is even considered to be “research” at all. This may seem like a simple or unnecessary question to consider, but many institutions have complex, specific definitions for research, and those definitions may be different across institutions and/or disciplines. Many institutional definitions of research focus on the use or involvement of human subjects in the research. If a project is determined by an IRB to not be research, it may not require IRB review at all.
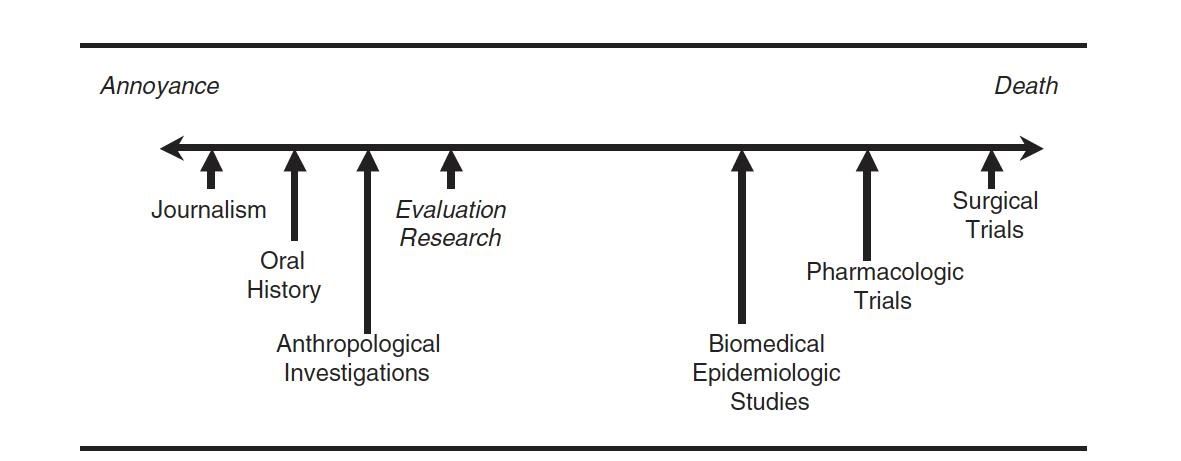
Common Modes of IRB Review
There are four common modes of IRB review:
- Exempt: Exempt from IRB review. Experienced reviewer must make the determination that a project is exempt (this usually requires submitting materials for review)
- Limited Review: Limited IRB review is required for research that meets specific expedited or exempt categories. This is new as of January 2019.
- Expedited: Protocol is reviewed by one IRB member, done on a rolling basis. Studies must fit into one of the 7 expedited categories.
- Full Board: Greater than minimal risk or doesn’t fit into an expedited category. These are the projects that go to a fully convened IRB.
Considerations for Evaluators
Hila offered a few helpful considerations for evaluators.
- Evaluation that’s part of a research study is reviewed together with that research study (cannot separate components of a research study) while evaluation of practice would be reviewed even if practice is not subject to review.
- If a project is not research or not human subjects research, it is still good practice to carefully consider consent content and process for collecting consent.
- Evaluators should look through the required elements of consent and think about what information you would like to have available if you were a participant. Recruitment processes, data protection, privacy and confidentiality are also helpful to think about.
- Take advantage of all of the resources that your IRB offers (may include guidance documents, meetings, educational sessions) and don’t be afraid to ask questions or request examples.
- Start early! Obtaining a determination or going through the review process will take time, there may be questions (about the proposed activities or evaluation more generally), and the details of the project may evolve.
Hila’s presentation slides can be found here (members only).